Neoantigen Dendritic Cell Vaccine Therapy
What is a Cancer Antigen?
When the immune system attacks cancer cells, the target it recognizes is called a cancer antigen. There are several types of cancer antigens, as outlined below:
- Shared Antigens: These are antigens that are highly expressed in cancer cells but are also expressed at low levels in normal cells.
- Neoantigens (i.e., Tumor-Specific Antigens): These are antigens that arise due to genetic mutations within cancer cells. They are uniquely expressed in cancer cells and not found in normal cells.
Antigens like WT1, which are shared antigens, are commonly expressed across many patients with the same type of cancer.
In contrast, neoantigens vary from patient to patient and are unique to each individual’s cancer.
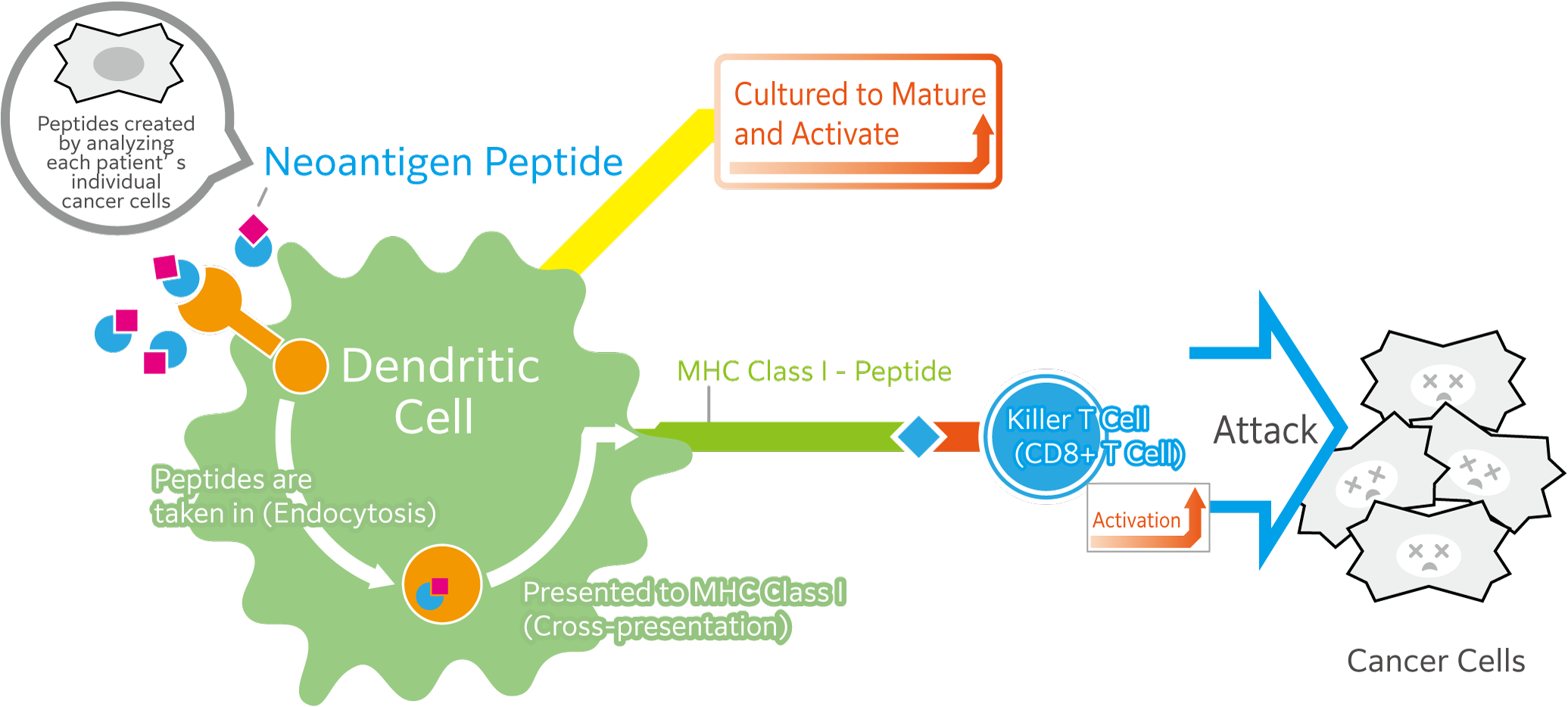
Advantages and Disadvantages of Neoantigen Dendritic Cell Vaccine Therapy
Conventional dendritic cell (DC) therapy involves introducing dendritic cells to shared cancer antigens—such as WT1 peptides—so that they can recognize these markers and pass the information to cytotoxic T lymphocytes (CTLs), thereby activating them to attack cancer cells.
Compared to this conventional method, using neoantigen-derived peptides (which are unique to each patient's cancer), the following advantages and disadvantages can be considered:
Advantages
To prevent the immune system from attacking normal cells, immune responses to antigens (markers) expressed on normal cells are typically suppressed by a mechanism called immune tolerance. Because of this, it is generally difficult to induce a strong immune response against shared antigens, which are expressed in small amounts on normal cells. However, since neoantigens are not expressed in normal cells, it is possible to induce a strong immune response against them.
Dendritic cell therapy using neoantigens is therefore considered to have a very strong ability to attack cancer cells. In fact, its high therapeutic effectiveness is being demonstrated in cancers such as melanoma.
Moreover, because neoantigens are expressed only in abnormal cells and not in normal cells, dendritic cell therapy that uses neoantigens is considered to have a lower risk of attacking normal cells, making it a safer treatment compared to dendritic cell therapy using shared antigens.
Disadvantages
Cancer tissue obtained through biopsy or surgery is required. Because neoantigens differ from patient to patient, it is necessary to use cancer tissue obtained from a biopsy or surgery to:
- Identify genetic mutations present in the cancer cells
(This is done by performing whole-exome sequencing using a device called a next-generation sequencer.) - Confirm whether the mutated genes are actually expressed
(This is done through RNA sequencing analysis using a next-generation sequencer.)
Both tests are necessary to identify neoantigens that may be useful for treatment. In particular, test (2) requires live cancer cells or cancer cells that have been frozen in a well-preserved state. Test (1) can be conducted using cancer tissue processed in hospitals (formalin-fixed paraffin-embedded samples), but if only test (1) is conducted, the accuracy of predicting useful neoantigens for treatment decreases.
Since these tests (1 and 2) are required in addition to conventional dendritic cell therapy, the overall cost increases. Furthermore, as of now, this treatment is not covered by insurance and is considered out-of-pocket (self-funded) treatment.